A mathematical model helps to explain how blood circulates in the brain
UC3M research analyses the appearance of oscillations in flow networks
2/2/22
Research carried out by the Universidad Carlos III de Madrid (UC3M) may help us better understand oscillations in blood flow that occur in the cerebrovascular network, thanks to a theoretical model that allows the flow and accumulation of fluid (in this case, blood) to be taken into account.
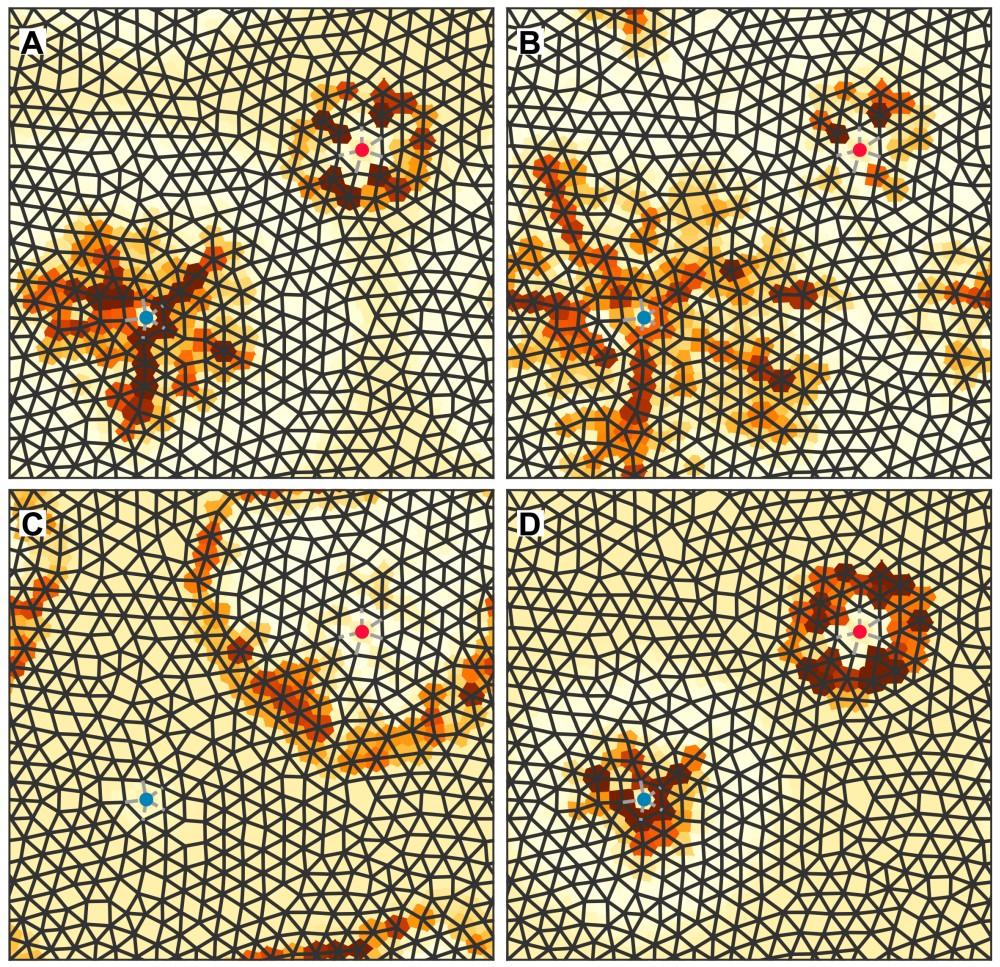
Travelling waves in a non-linear flow network. Each panel displays different time frames. The network is represented by black lines while the colour around each node indicates the volume that has accumulated at that node. The red and blue nodes maintain a constant pressure difference (similar to connecting them to a pressure pump). The way in which the wave begins to form around the blue node, how it grows and travels to the red node can be observed. This process is repeated periodically, resulting in oscillations. Credit: Miguel Ruiz García and Eleni Katifori.
Flow networks are made up of a set of connections that carry fluid. The current that circulates through these “ducts” usually increases if the pressure difference between the inlet and outlet increases. However, in certain non-linear flow networks, such as the circulatory system, the current may fall as the pressure difference increases. “This behaviour is known as negative differential resistance and has been observed in blood vessels and devices that conduct electricity,” says one of the researchers, Miguel Ruiz García, a CONEX-Plus researcher at the UC3M’s Department of Mathematics.
Blood vessels are more like active organs than rigid ducts. Specifically, the arteries are covered by vascular musculature that allows them to contract or expand in response to different stimuli. For example, when a blood vessel that feeds an organ detects an increase in pressure at its inlet, it can respond to that increase in pressure by contracting (compressing its muscles) in order to reduce flow and protect the organ. “This effect is called the myogenic mechanism, and there are similar effects that cause the flow through a blood vessel, which is not a linear function of the pressure difference, but a non-linear function that sometimes has a negative differential resistance,” notes Miguel Ruiz García.
This theoretical model, which allows the size of the network to be estimated using a method that takes the connections between ducts into account and predicts the frequency of pressure oscillations, was recently presented at the International Conference on Complex Networks and their Applications. “We were able to observe interesting phenomena, such as the appearance of waves that travel through these complex networks. It turns out that the frequency of these oscillations changes as we change the structure of the network in very different ways. Explaining why these different structural changes lead to similar changes in frequency was very challenging and we were only able to do it using a topological metric: a value that measures the “effective” size of the network,” explains Miguel Ruiz García.
They are called topological metrics because they use the network’s topology, in other words, they take their internal connections into account. “We can measure, for example, the distance between cities in kilometres and say that Madrid is closer to Teruel than to Barcelona. However, if we measure the distance as 1 divided by the number of trains that travel from Madrid to each of these cities on a daily basis, then Barcelona is much “closer” than Teruel according to our new way of measuring. This type of measure provides us with information about the difficulty of travelling from one point to another within the network,” says the researcher. “Similarly, the topological measure we use tells us the effective size of the system, so if the system is smaller effectively then waves take less time to get from one end to another and their frequency increases. This is similar to the previous example, in which it is easier to get to Barcelona than Teruel,” he concludes.
“Our theoretical results could help other researchers better understand the oscillations that are observed in blood that circulates in our brain, as these blood vessels present the conditions that our model is studying,” says Miguel Ruiz García. “On the other hand,” he continues “we hope that our experimental work will develop new devices that help control flow in microfluidic devices (devices with very small pipes that are used in the pharmaceutical industry, as well as in many laboratory devices)”.
This piece of research began when Miguel Ruiz García was working as a post-doctoral researcher at the University of Pennsylvania (USA), along with lecturer Eleni Katifori. This research has been able to be developed thanks to his incorporation into the UC3M as a CONEX-Plus talent attraction programme researcher, funded by the University and the European Commission through the Marie Skłodowska-Curie COFUND Actions (GA 801538) from the European Horizon 2020 Framework Programme.
More information:
Ruiz-Garcia, M. Katifori, E. (2021). Topology controls the emergent dynamics in nonlinear flow networks. The 10th International Conference on Complex Networks and their Applications. November 30 - December 2.. Madrid, España. https://complexnetworks.org/
Ruiz-Garcia, M. Katifori, E. (2021). Emergent dynamics in excitable flow systems, Physical Review E 103 (6), 062301. https://doi.org/10.1103/PhysRevE.103.062301
--------------------